Video overview of mRNA vaccine technology

This article was originally published here.
This 25-minute video explains in detail how the COVID-19 mRNA vaccine works, as well as highlights some of the known risks. If you want to better understand technology or want to improve your knowledge of technology, as well as learn a little about molecular and cellular biology – this is a great primer. So sit back, relax, and open your mind to a little science.
Analysis of peer-reviewed research
Neutralize antibodies against the SARS-CoV-2 variant Omicron (BA.1) 1 to 18 weeks after the second and third doses of the BNT162b2 mRNA vaccine. JAMA Netw Open.In 2022; 5 (5): e2212073. doi: 10.1001 / jamanetworkopen.2022.12073
The abstract of the paper mentions for the first time that in initial clinical trials, SARS-CoV-2 neutralizing antibodies were correlated with protection against infection and disease caused by viral variants. original withdrawal caused, but not mentioned by the authors – this correlation has not been demonstrated with Omicron variants.
The authors of this present work, focusing on the Pfizer/BioNTech vaccine, note that in studies conducted by Moderna, a decrease in neutralizing antibody titers was observed in the vaccinated population. , which corresponds to a decrease in vaccine efficacy against polymerase chain reaction – confirmed Omicron infection in Denmark and symptomatic Omicron infection in the United Kingdom.
The study had an odd selection bias – it included only men, and it did not collect or analyze any data regarding efficacy or efficacy endpoints.
This study repeatedly confirms that there is a proven correlation between neutralizing antibody titers and protection against infection and disease, but is based on historical data from non-Omicron variants to support this claim.
- This study detected a rapid decrease in the Omicron-specific neutralizing antibody titer just a few weeks after the second and third doses of BNT162b2.
- For persons 65 years of age and older, there was virtually no Omicron-specific serum neutralizing antibody titre after dose 2 (see Figure 2, panel B).
- For subjects 65 years of age and older, there was virtually no serum Omicron-neutralizing antibody titre at week 8 after dose 3.
Please review Figure 2, which clearly shows these results:
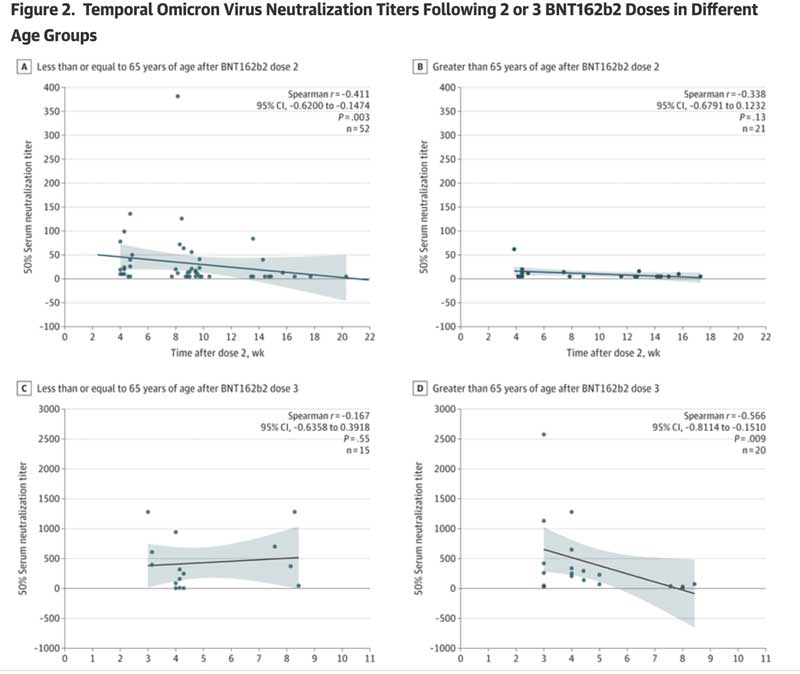
Taken together, this work clearly demonstrates that protective vaccine-induced antibody responses following the second and third doses of BNT162b2 are both very low and transient in older adults (who are at-risk populations). the most significant risk of hospitalization and death).
The article mentions that conserved T-cell immunity and non-neutralizing antibodies can still protect against hospitalization and death even with attenuated neutralizing antibodies, but the study didn’t actually measure T-cell immunity and non-neutralizing antibodies.
The authors concluded that “additional booster doses may be necessary, especially in older individuals.” By their logic, one could look at the table above and note that the extent of protection rapidly declines after week 4 in people over 65 years of age after dose 3. One has to wonder, whether the study authors does it really imply that a total of 13 boosters per year is needed (52 weeks / four weeks = 13 boosters)? Or are they just writing this for peer review?
Also relevant is that “confirmed correlation of protection” is a precise regulatory term. It means extensive analysis and characterization of both the specific test used (neutralizing antibodies against SARS-CoV-2), as well as the relationship between what it is testing and which it is being developed to replace (protection ) already done. In the case of the present study, this did not happen.
Therefore, no conclusions can be drawn from this study regarding what it is measuring (targeted neutralizing antibody) and the endpoint it is intended to replace (protection) with. can be drawn. In other words, this paper is essentially a preliminary study with no real legal implications – it has not been conducted with the rigor required.
A more non-objective conclusion based on these data is that the Pfizer/BioNTech BNT162b2 vaccine has particularly poor stability to antibody neutralization (using an unconfirmed “academic study” level test). real) against circulating virus strains..
In contrast, efficacy data for emerging mRNA vaccines from many countries consistently demonstrate a dose-dependent negative effect on these vaccines (in other words, the more doses administered, the lower the risk of disease). or significantly higher COVID deaths).
Failure to address these data again clearly demonstrates both the failure of the peer review process, the authors’ irrational vaccine bias, and the pro-vaccine bias. by the editors of the Journal of the American Medical Association.
When can objective, data-driven physicians and medical scientists restore their previous confidence in the Journal of the American Medical Association, or will JAMA continue to uphold (for all practical purposes) just a trade magazine advertising the pharmaceutical industry?