Using excess heat to improve electrolytes and fuel cells
New technology can help create hydrogen and chemical industry components.
Reducing fossil fuel use will have unintended consequences for the electricity generation industry and beyond. For example, many industrial chemical processes use fossil fuel by-products as precursors to things like asphalt, glycerine, and other important chemicals.
One solution to reduce the impact of fossil fuel loss on industrial chemical processes is to store and use the heat generated by nuclear fission. New MIT research has dramatically improved a way to put that heat into making chemicals through a process called electrolysis.
An electrolyzer is a device that uses electricity to separate water (H2O) and produces hydrogen molecules (H2) and oxygen (O2). Hydrogen is used in fuel cells to generate electricity and control electric cars or drones, or in industrial activities such as making steel, ammonia and polymers. The electrolyzer can also take in water and carbon dioxide (CO2) and produces oxygen and ethylene (C2H4), a chemical used in polymers and elsewhere.

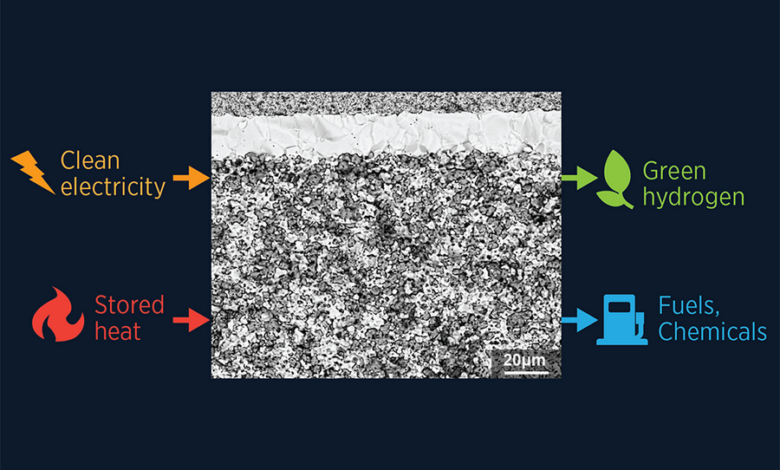
Note: Clean electricity and stored heat from nuclear and intermittent electricity from wind and solar can be efficiently converted into green hydrogen, fuel and chemicals using a protonic ceramic electrolyzer with high efficiency. outstanding performance and stability. Illustrated by researchers / MIT
There are three main types of electrolyzers. One that works at room temperature, but has disadvantages; they are inefficient and require rare metals, such as platinum. The latter is more efficient but runs at high temperatures, above 700 degrees Celsius. But metal corrodes at that temperature, and devices require expensive insulation and insulation.
A third type would be the Goldilocks solution for nuclear heat if it was perfected, runs at 300-600 C and requires mostly inexpensive materials like stainless steel. These cells have never been run as efficiently as the theory says. New work, published in Natureboth clarifying the problem and providing the solution.
A sandwich mystery
Intermediate-temperature devices use what are known as proton-ceramic electrochemical cells. Each cell is a sandwich, with a dense layer of electrolyte sandwiched between two porous electrodes. Steam is injected into the top electrode. A side wire connects the two electrodes and an externally generated current flows from top to bottom.
The electric potential pulls the electrons out of the water, splitting the molecule, releasing oxygen. A hydrogen atom with no electrons is just a proton. The protons are pulled through the electrolyte to re-bond with the electrons at the bottom electrode and form H2 molecules, which are then collected.
The middle electrolyte alone, made mainly of barium, cerium and zirconium, conducts protons very well. “But when we put the same material into this three-layer device, the proton conductivity of the entire cell,” said Yanhao Dong, a postdoc in MIT’s Department of Nuclear Science and Engineering and a co-author of the paper. cell is pretty bad. “Its conductivity is only about 50% of the bulk form. We wonder why there is an inconsistency here.”
A few clues pointed them in the right direction. First, if they don’t prepare the cells very carefully, the top layer, which is only about 20 micrometers (0.02 mm) thick, won’t stick. “Sometimes if you just use Scotch tape, it just comes off,” says Dong.
Second, when they examined the cross-section of a device with a scanning electron microscope, they found that the top surface of the electrolyte layer was flat, while the bottom surface of the porous electrode rested on it. bumpy, and the two have come into contact only in a few places. They don’t link well. That precarious interface leads to both structural delamination and poor movement of protons from the electrode to the electrolyte.
Acid solution
The solution turned out to be simple: the researchers roughened the top of the electrolyte. Specifically, they applied acid for 10 minutes to carve the grooves into the surface. Ju Li, Battelle Energy Alliance Professor of Nuclear Engineering and professor of materials science and engineering at MIT and co-author of the paper, likened it to sandblasting a surface before painting. to increase adhesion. Their acid-treated cells produce about 200% more hydrogen per area at 1.5 volts at 600 C than any previous cell of the same type and perform well at 350 C. with very low performance under prolonged operation.
“The authors reported a surprisingly simple yet highly effective surface treatment method,” said Liangbing Hu, director of the Center for Materials Innovation at the Energy Innovation Institute of Maryland, who was not involved in the study. course to significantly improve the interface. He calls the cell’s performance “exceptional.”
“We are delighted and surprised” by the results, Dong said. “The technical solution seems quite simple. And that’s really good, because it makes it very applicable to real-life applications. ”
In a real product, many such cells will be stacked to form a module. MIT’s partner on the project, Idaho National Laboratory, is strong in engineering and prototyping, so Li hopes to see electrolyzers based on this technology on scale sooner. “At the materials level, this is a breakthrough showing that at real device scale you can work at the sweet temperature of 350 to 600 degrees Celsius for fusion reactors and nuclear fission.
“The reduced operating temperatures make materials cheaper for large-scale assembly, including stacking,” said Dong Ding, Idaho National Laboratory researcher and paper co-author. “The technology operates in the same temperature range as several important industrial processes today, including ammonia and CO production.2 palliation. Adapting to these temperatures will accelerate the adoption of the technology in current industry. “
“This makes a lot of sense for both Idaho National Lab and us,” added Li, “because it bridges the gap between nuclear power and renewable electricity.” He notes that the technology could also help fuel cells, which are essentially reverse-engineered electrolyzers, to use hydrogen or green hydrocarbons to generate electricity. According to Wei Wu, a materials scientist at Idaho National Laboratory and a co-author of the paper, “the technique is quite common and compatible with other solid electrochemical devices.”
Dong says it’s rare for a paper to advance both science and technology to such an extent. “We’re happy to put those things together and get a very good scientific understanding and very good practical performance as well.”
Written by Matthew Hutson
The source: Massachusetts Institute of Technology



