Climate Change, an Emergency, or Not? – Watts Up With That?
The Impact of CO2, H2O and Other “Greenhouse Gases” on Equilibrium Earth Temperatures
By David Coe
The cries of Climate Emergency are becoming ever more strident. We are bombarded on a daily basis from almost every section of the media with stories of impending doom unless we take immediate and decisive action to prevent a climate catastrophe. This action includes the rapid adoption of a “zero carbon economy”. But what does this actually mean?
Carbon is the atom which, above all others, is the basis of life on this planet. Its unique atomic structure enables it to combine with other atoms to produce the amazing variety of complex molecules necessary for all forms of life.
Of course ,what is meant by “zero carbon” is actually zero carbon dioxide, the molecule which has been “fingered” as the demonic source of global warming, now known as climate change, climate emergency, climate catastrophe or whatever the next superlative tag can be attached to the word climate.
Just how does carbon dioxide come to be the instigator and chief cause of global warming? I say chief cause, because other gases are also in the frame as contributors, namely methane and nitrous oxide, for which the global agricultural sector is shouldering the blame, because of methane liberated by cattle and nitrous oxide from fertilisers. The story goes like this.
The atmosphere contains 400 parts per million (ppm), 0.04%, of carbon monoxide (CO2) which is known to be a strong “greenhouse gas”. A “greenhouse gas” is one which is transparent to incoming solar radiation, but which is a strong absorber of outgoing infra-red energy radiated by a warming earth. The more CO2 in the atmosphere, the more outgoing radiation is absorbed and the warmer the planet gets. Simples!
Carbon Dioxide
Carbon dioxide just happens to be the vehicle for providing the carbon for the production of the complex organic molecules necessary for life, through the process of photosynthesis in plants which in turn provide the feedstock for all other forms of life. Without CO2 in the atmosphere there will be no life on earth. Photosynthesis extracts huge quantities of CO2 from the atmosphere on a seasonal basis. As a result, atmospheric CO2 concentrations exhibit a significant seasonal variation as shown in Figure 1, with the effect that CO2 levels undergo a rapid reduction in the northern hemisphere in the spring and summer as vegetation awakes from its winter slumbers and bursts into life with massive regrowth from the CO2 sequestered from the atmosphere.
In addition to this seasonal variation there is an underlying persistent increase in CO2 levels, which has been attributed to the release of CO2 into the atmosphere from the combustion of fossil fuels which have powered the industrial economies since the start of the industrial revolution some 200 years ago. Since that time CO2 levels have risen from 280ppm to over 400ppm. It is also worth noting that the seasonal variation itself increases with latitude so that the seasonal variations in the arctic circle are some 10 times greater than the annual increase attributed to the combustion of fossil fuels, underscoring the role that nature plays in the atmospheric presence of CO2.
Figure 1:- annual variations in CO2 concentrations at latitudes up to 82°N

It is this annual increase, however, which is fuelling the concerns over the warming of the planet. At first sight these concerns appear to be well founded and should not be dismissed. Equally, neither should the economic consequences of a “zero carbon economy” be ignored. It was absolutely right therefore for the United Nations to take a lead in determining the exact nature of the causes and implications of “anthropogenic global warming” by setting up the “Intergovernmental Panel on Climate Change” (IPCC) in 1988. Their mandate was and still is to identify the evidence to support the concept of global warming and seek methods to mitigate its impact.
The IPCC set about its task with exemplary determination in seeking evidence to support the concept of anthropogenic warming, while, unfortunately, studiously ignoring any and all evidence which might suggest an alternative narrative. There is thus an inbuilt bias in the terms of the IPCC and certainly in the manner in which it operates.
After some thirty years of extensive efforts by thousands of climate scientists around the world and the expenditure of billions of dollars, granted to universities and others for the research work, there is still considerable uncertainty about the impact on global temperatures of the so called “greenhouse effect”. This is summed up neatly by the UK MET office in its website “What is Climate Sensitivity?”, in referring to Equilibrium Climate Sensitivity (ECS), which is a parameter invented by the IPCC to represent the increase in average global temperature caused by a doubling of the atmospheric CO2 concentration.
“As there is no ‘perfect’ way of estimating climate sensitivity, it remains a hotly debated area of science and there remains a wide range of estimates of what the ECS could be.”
In fact, estimates of climate sensitivity throughout the years have varied between 1°C to over 6°C, settling down at this moment in time to a band between 1.5 and 4.5°C, still a factor of three variation. Why is there such uncertainty? Well, the atmosphere, while a relatively thin layer of gas around 50km thick, is an incredibly complex entity, often described as a non-linear chaotic system.
The Greenhouse Effect
The composition of the atmosphere has varied over history, but at this time comprises nitrogen(77%), oxygen(21%), argon(1%), Water vapour 1%, CO2 0.04% (400parts per million) with trace levels of methane (1.8parts per million) and nitrous oxide (0.32parts per million). Of these gases CO2, water vapour, methane and nitrous oxide are considered to be greenhouse gases. That is, they absorb some of the infra-red energy being radiated by the earth into space, while freely transmitting solar energy down to the earth’s surface.
The earth’s average temperature is determined solely by the energy balance at the top of the atmosphere. The radiation emitted by the earth is a function of its temperature. The warmer the earth, the more radiation it emits. When the energy radiated by the earth into space is equal to the solar energy received from the sun, the earth temperature will have reached equilibrium and will be stable. When some of this radiated energy is absorbed by the atmosphere, the energy balance is disturbed, and the earth warms in order to restore the balance. The question is, by how much?
Effective Earth Temperature
The starting point for this question is, what would be the temperature of the earth if no atmosphere existed and the earth’s radiation was emitted through to space without any absorption? From a knowledge of the intensity of solar radiation received by the earth and the infra-red radiation emissions as a result of the earth’s temperature, it is widely accepted that the average earth temperature would be a chilly -18°C.
Atmospheric Absorption
The current average earth temperature is generally reckoned to be a comfortable +15°C and so the total impact of the atmospheric greenhouse effect is to produce a warming of 33°C. What we need to know is precisely the impact of each of the “greenhouse gases”, particularly that of CO2. How do they each contribute to this warming? The answer to this requires a detailed knowledge of the infra-red absorption characteristics of these gases.
We are, therefore, fortunate to have HITRAN, a free access data base of molecular spectrographic data available to us. HITRAN was first introduced almost 50 years ago and has developed since then, particularly over the past 20 years, into the foremost repository of gaseous molecular spectra. From this dataset we can now calculate with high precision the radiation absorption characteristics of these “greenhouse gases”.
First, however, it is necessary to know the nature of the radiation emitted by the earth. All bodies radiate energy, the hotter the body, the higher the emitted radiation intensity. Figure 2 shows the intensity and wavelength of the radiation emitted by the earth at its current average temperature of 15°C. This radiation spectrum extends from the near infra-red (3micron) to almost microwave (100micron) wavelengths. Micron is a common unit of radiation wavelength equal to one millionth of a metre. Visible light, for example, extends from blue light at 0.4micron to red light at 0.65micron, and is emitted in copious amounts by the sun as a result of its high temperature in excess of 5000°C. The 15°C temperature of the earth results in emitted radiation at much longer wavelengths up to 100micron.
Figure 2

Absorption due to CO2
Figure 3 shows the transmission of the earth’s emitted radiation through the current 400ppm of atmospheric CO2 across the radiation emission spectrum shown in Figure 2.
Figure 3
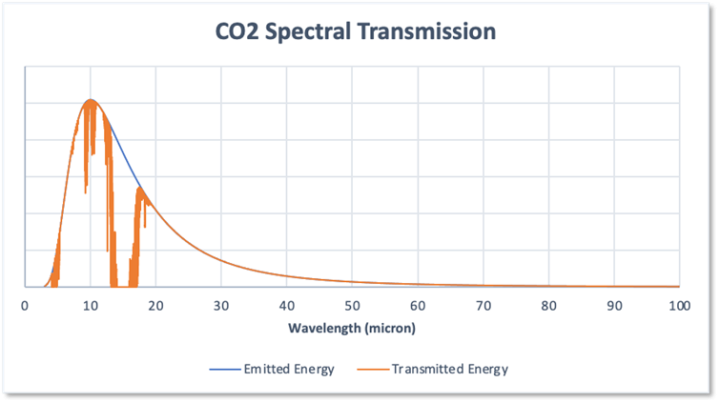
The small amount of atmospheric CO2 takes a large slice out of the emitted radiation, absorbing some 18.7% of the total radiated energy.
Absorption due to Water Vapour
The most abundant greenhouse gas is water vapour. The concentration of water vapour in the atmosphere, unlike the other greenhouse gases, is determined solely by temperature and pressure, taking it out of the influence of mankind. Figure 4 shows the spectral transmission of the radiated energy to space through the atmospheric water vapour.
Figure 4

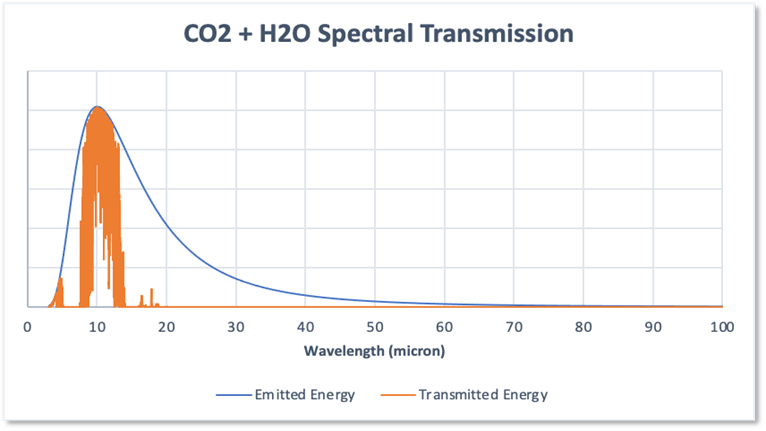
Water vapour takes an absolutely huge bite out of the radiated energy, absorbing 67% of that energy, including all energy at wavelengths beyond 20micron. This would imply that the combined absorption due to CO2 plus water would be 18.7% + 67.0% = 85.7%. This would also be wrong!
If you compare Figures 3 and 4 it will be apparent that the absorption bands of the two gases overlap to a large degree. In particular, because the water spectrum dominates, the impact of CO2 is much reduced so that when the two spectra are combined the absorption is as shown in Figure 5. In effect the two gases fight over the common absorption wavelengths, and of course due to its much higher abundance, water vapour wins.
Figure 5
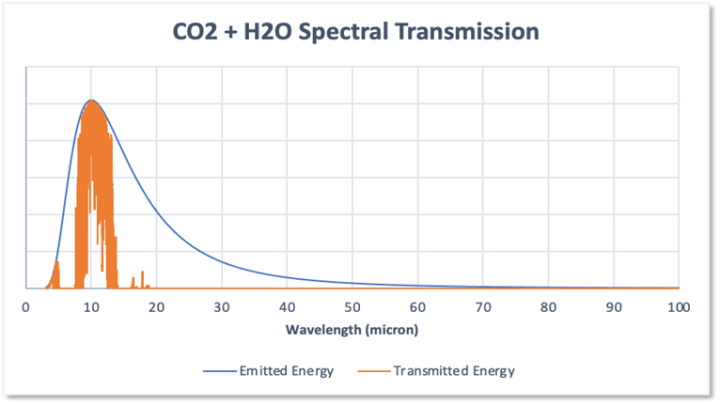
The result is that the total absorption by the combination of CO2 and water vapour is 72.6% and the impact of CO2 on absorption is thus to increase the absorption due to water alone from 67% to 72.6% an increase of just 5.6% not the 18.7% due to CO2 on its own. This has a major bearing on the role of CO2 with respect to its global warming potential.
Absorption due to Methane
The role of methane is important, not simply because it is a “greenhouse” gas, but because it has been identified as a “major contributor” to climate change, to the point that there is now pressure to refrain from eating meat and to adapt to a vegetarian diet. This is on the basis that farm animals, and cattle in particular, are major emitters of methane. It would therefore be quite interesting to determine exactly the contribution made by methane to the atmospheric absorptivity.
Figure 6 shows the transmission of radiation through the 1.8parts per million (ppm) of methane currently in the atmosphere.
Figure 6

It has a much smaller absorption profile than CO2 providing a total absorption of 1.6%. So much for the erroneous claims that methane is 100 times more powerfull a greenhouse gas than CO2. As with CO2, the absorption bands overlap with those of water vapour and also those of CO2, so that the impact of 1.8ppm of methane increases the total absorption from 72.6% to 72.8%, an increase of only 0.2% The inescapable conclusion is that current methane levels have minimal impact upon atmospheric radiation absorption.
Absorption due to Nitrous Oxide
Atmospheric nitrous oxide concentrations at 0.32ppm are the lowest of the four commonly encountered greenhouse gases. Its absorption spectrum is limited to a small region of the radiation transmission as seen in Figure 7 with an absorption of 1.7%, similar in fact to the absorption of current levels of methane. Its absorption bands, also like methane, are overlapped by those of water vapour and CO2, resulting in a contribution to absorption also of 0.2% bringing the total atmospheric absorption of radiated energy to 73.0%.
Figure 7

Resultant Atmospheric Absorption
The combined total effect of greenhouse gas absorption is put into perspective in the pie chart of Figure 8, demonstrating the dominance of water vapour in the absorption of emitted radiation.
Figure 8

Section 1 blue – Absorption by CO2 5.6%
Section 2 orange – Radiation transmitted through the atmosphere 27.0%
Section 3 grey – Absorption by Water Vapour 67.0%
Section 4 yellow – Absorption by Methane + Nitrous Oxide 0.4%
It is clear from this chart that water vapour is by far and away the most powerful absorber of the earths radiated energy.
Absorbed Radiation – where does it go?
So far the data presented on the absorptivity of greenhouse gases is based upon the well documented gaseous spectral data in the HITRAN database and known atmospheric composition, without any conjecture or assumption.
The issue now is to compute the impact on global temperature of this atmospheric absorption of energy. First, however, we must answer one vitally important question. What happens to the 73% of radiated energy absorbed by the atmosphere?
It is not unreasonable to believe that the atmosphere itself will warm as a result of this energy input, and if so, will reradiate some of this energy into space, while the remainder will be retained by the earth thereby warming it. The question then is – how much of the absorbed energy is ultimately retained by the earth? Failure to provide an accurate answer to this question has resulted in the wide variation in estimates of the climate sensitivity to CO2 and uncertainty in the predictions of global warming to the present date. Attempts to answer this by computer modelling the complex atmospheric processes, while proving very lucrative for an army of climate scientists and universities over several decades, have been essentially futile.
There is, though, despite the pronouncements of the UK Met Office, a simple method for the determination of retained energy that requires no knowledge of, or assumptions about, the complex atmospheric processes, that will enable us to accurately determine the climate sensitivity of all greenhouse gases.
Current Earth Temperature
We know the solar energy reaching earth. It is the same value as used to calculate the Effective Earth Temperature of -18°C, with no atmosphere. It is also generally agreed that the average earth temperature is 15°C or thereabouts. From that temperature it is possible to calculate the amount of energy radiated by the earth. It is in fact the sum of the energies over the spectral range 3 to 100 micron shown in Figure 2. Now some of that energy will be absorbed and retained by the atmosphere. The rest will be transmitted through to space. It is a simple calculation, using the energy balance at the “top of the atmosphere” to show that, in order to maintain a temperature of 15°C only 61.5% of the radiated energy will be transmitted to space. Thus, a total of 38.5% of the radiated energy must be absorbed and retained by the atmosphere/earth.
This is not conjecture. It is a simple fact.
However, we have determined that the atmosphere currently directly absorbs 73% of the outgoing energy, therefore it is a further simple calculation to realise that only 52.7% of this absorbed energy is actually retained by the earth and its atmosphere (52.7% of 73% = 38.5%).
This figure of 52.7% of absorbed energy retained does not differentiate between which gases are responsible for the energy absorption. It applies equally to all the absorbed energy no matter which gas is responsible. This enables us to determine how much energy absorption and retention can be attributed to each greenhouse gas. It is simply 52.7% of the absorption values shown in Figure 8.
We can now look at the total radiation transmission and absorption budget in Figure 9

Figure 9
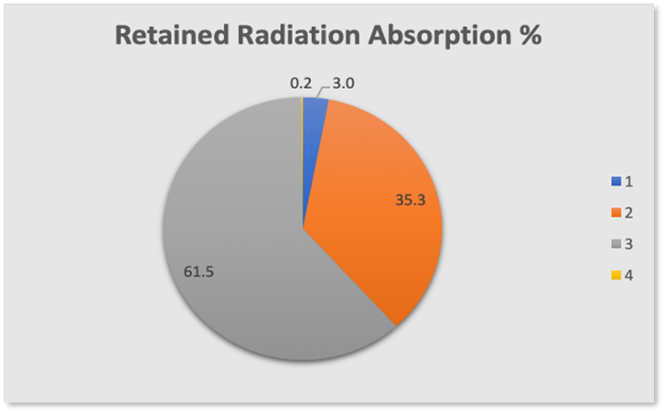
Section 1 blue – Absorption by CO2 3.0%
Section 2 orange – Absorption by Water 35.3%
Section 3 grey – Total Radiation transmitted through to space 61.5%
Section 4 yellow – Absorption by methane + nitrous oxide 0.2%
We see immediately that only 3% of the energy radiated by the earth is actually absorbed and retained by the CO2 in the atmosphere.
Impact on Temperature
The total energy absorbed and retained by the atmosphere (38.5%) produces the warming of 33°C to provide us with the current temperature of 15°C. Because we now know the absorption contribution for each of the greenhouse gases we can allocate their individual contribution to the 33°C warming ( Figure 10 ).
Figure 10
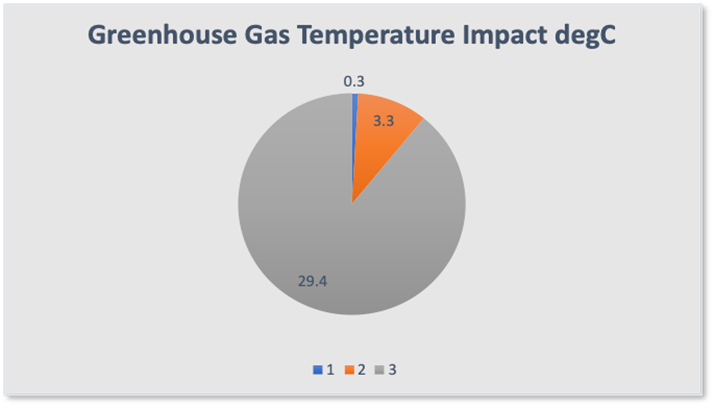
1 Blue – Methane + Nitrous Oxide 0.3°C
2 Orange – CO2 3.3°C
3 Grey – Water Vapour 29.4°C
Some 90% of the current warming, a total of 29.4°C can be directly attributed to water vapour. CO2 contributes just 3.3°C while the combined impact of methane and nitrous oxide is a barely measurable 0.3°C.
Not only can we attribute the present warming to individual greenhouse gases, we now have a method of predicting the warming resulting from increasing levels of individual greenhouse gases from their respective infra-red absorption spectra and the HITRAN database.
Warming due to the Increase in CO2 from 280 to 420ppm
It is believed that prior to the industrial revolution, atmospheric CO2 levels were typically 280ppm. Since then CO2 levels have increased to 420ppm. From the absorption spectra we can calculate that the absorptivity of the atmosphere will have increased from 72.7% to 73.0% during that period, due to this increase in CO2 levels. The increase in temperature resulting from that increased absorption of energy is 0.24°C. It is generally accepted that over this period the earth has actually warmed by around 1°C. It is therefore completely wrong to attribute this increase totally to anthropogenic global warming. Only 25% of that warming can be attributed to the increase in CO2 levels. More and more evidence is coming to light, that the earth undergoes regular variations (in geological timescales) in temperature, unrelated to atmospheric CO2 levels, possibly linked to the earth’s primary source of energy, the sun.
Warming Due to Future Increases in CO2 Concentrations
The following graph (Figure 11) shows the increase in absorptivity if CO2 concentrations were to increase up to 1600ppm, some 4 times higher than current concentrations. As can be seen from the graph, increasing CO2 concentration has only a small impact on total absorptivity, because of the near complete absorption of the emitted radiation corresponding to the greenhouse gas absorption bands. Adding more and more CO2 to the atmosphere has less and less influence on atmospheric absorption and hence global temperatures. This massive increase in CO2 would increase the atmospheric absorption from 73% to just 74.6% of the earth’s radiated energy.
Figure 11

This would result in an increase in temperature of just 1°C ( Figure 12 ).
Figure 12

Climate Sensitivity to CO2
Climate sensitivity is the measure of the impact of greenhouse gases introduced by the IPCC. It gives a value for the temperature increase caused by a doubling of greenhouse gas concentrations. This value may be deduced for CO2 from the previous graph of earth temperature v CO2 concentration. At current CO2 concentrations the climate sensitivity for a doubling of CO2 concentration from 400 to 800ppm is just 0.45°C implying an average earth temperature of 15.45°C when CO2 levels reach 800ppm, which at the current rate of increase of atmospheric CO2 concentration of approximately 1.5ppm per year (see Figure 13), will not occur for another 250 years. So much for the current climate emergency and hysteria.
Figure 13

Climate Sensitivity to Methane and Nitrous Oxide
The media are full of reports on how climate change can be tackled by restricting meat consumption on the basis that farm livestock, particularly cattle emit methane from their digestion of grasses. It is interesting to note exactly what the climate sensitivity is for methane. This is shown in the graph below (Figure 14).
Figure 14

Doubling the current methane concentration from almost 2ppm to 4ppm will increase global temperatures by 0.06°C. In the face of this, just how many people would be prepared to switch from eating beef to eating insects in order to “save the planet”, particularly when methane increases are limited by the natural oxidation of methane into carbon dioxide and water vapour within the atmosphere.
The climate sensitivity to nitrous oxide is little different
Figure 15

Doubling the current levels of nitrous oxide will increase temperatures by a total of 0.08°C.
These levels of potential temperature increase are so small as to be almost unmeasurable. Yet according to the all-knowing cadre of climate scientists and fawning media they pose an existential threat to the climate and to our future.
Climate Feedback Effects
Of course, when presented with data that suggests that there is no problem, the climate change enthusiasts will enlist the claim that the warming caused by these gases will be amplified by the feedback effects of water vapour. As stated earlier the concentration of water vapour in the atmosphere is dependent uniquely on atmospheric temperature. As temperature increases, the concentration of water vapour will also increase, and that increase will in turn increase atmospheric absorption and hence increase further the temperature in a never-ending cycle. It is easy to argue that this process will ultimately lead to a “tipping point” and runaway temperatures. A real climate emergency!
It is always useful to argue from a basis of fact.
Fact 1 Water vapour concentration exists predominantly in the lower atmosphere because of its relationship to temperature and pressure. Temperature and pressure both reduce with altitude, and so does water vapour. This is clearly seen in the accompanying graph in Figure 16.
Figure 16
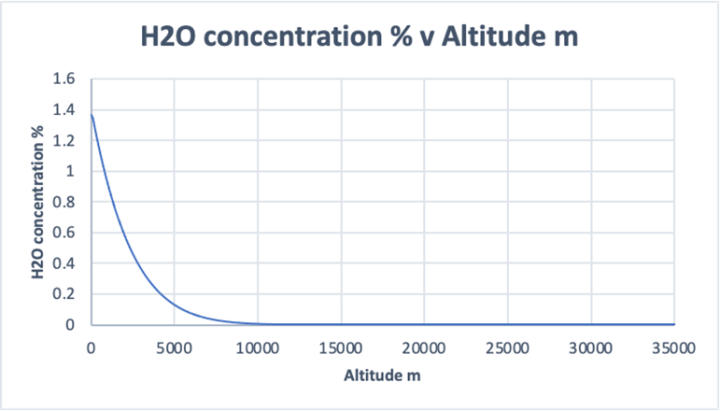
Very little water vapour exists above 10km altitude.
Fact 2 The rate at which water vapour concentration (expressed as Saturation Vapour Pressure SVP) increases with temperature also varies with altitude, tailing off to almost zero above 10km. The largest change occurs at sea level and is typically 0.088% concentration per °C of temperature increase (Figure 17).
Figure 17

Fact 3 Water Vapour is a very, very powerful absorber of infra-red radiation. Even if the water vapour were to reduce to a tenth of its current atmospheric level the atmosphere would still absorb over 50% of the earth’s radiation (Figure 18). Paradoxically if water vapour was to increase by 20% from its existing level to a level of total atmospheric saturation the atmospheric absorption would increase only slightly from its current 73.0% to a value of 73.9% resulting in a temperature increase of just 0.5degC. This is because small amounts of water vapour quickly absorb most of the radiation in its absorption bands leaving very little effect for further increases in water concentration and consequently little effect on temperature.
Figure 18
Fact 4 From these known characteristics of water vapour, the water vapour feedback effect would not lead to a runaway temperature but would result in an increase in a temperature of 1°C being increased by 12% to a temperature of 1.12°C. There is not, and never can be, a climate tipping point. The earth, to a large extent, owes its temperature stability to the characteristics of water vapour over which we have absolutely no control or influence. Large variations in water vapour concentrations have relatively little effect upon atmospheric absorption and hence earth temperatures.
Conclusions
As a direct consequence of the greenhouse effect the earth is 33°C warmer than it otherwise would be. Without the greenhouse gases to warm the earth we would not be around to fret about the consequences. Of the 33°C warming, 29.4°C is entirely due to the absorptive effects of water vapour. 420ppm of CO2 delivers just 3.3°C of that warming, while methane and nitrous oxide are responsible for a mere 0.3°C combined.
Contrary to the blitz of propaganda, there is no climate emergency or even any significant increase in temperature due to increasing levels of CO2. The climate sensitivity to a doubling of CO2 is 0.45°C which increases to 0.5°C when the feedback of water vapour is taken into account. A four-fold increase in CO2 concentrations to 1600ppm will increase temperatures by 1°C and it would take around 800 hundred years to reach that point at the current rate of CO2 level increases. It would however offer multiple beneficial effects, such as increased crop yields and greening of desert areas. The adoption of a zero-carbon economy, at a cost of not just billions of dollars, but trillions, will have no discernible effect upon the climate whatsoever, even assuming that all nations would adopt such a policy. The IPCC pronouncements, which form the basis for the headlong stampede to “zero carbon” are simply wrong. Their estimates of climate sensitivity are out by a factor of at least three and possibly ten!
The fearmongering over methane emissions from cattle is just that. The climate sensitivity to a doubling of methane is just 0.06°C. And for this we are asked to restrict the consumption of beef and even replace it with insects and mealworms. No thank you!
Variations of earth temperature of many degrees Celsius, over millennia, are known to have occurred caused by entirely natural phenomena, particularly solar radiation intensity variations. The medieval warm period and little ice age are two recent examples. Scientific concern could perhaps be better focussed on the possibility, ne probability, that we are approaching the end of an interglacial period at which point the earth will enter a new ice age. Our impotance to influence the climate will then be clearly and painfully realised.
The data for this article is derived from the paper “The Impact of CO2 and Other Greenhouse Gases on Equilibrium Earth Temperature” published in the International Journal of Atmospheric and Oceanic Science.
The link to the paper is http://www.ijaos.org/article/298/10.11648.j.ijaos.20210502.12 .