Scientists discover new therapeutic target to treat colorectal tumors
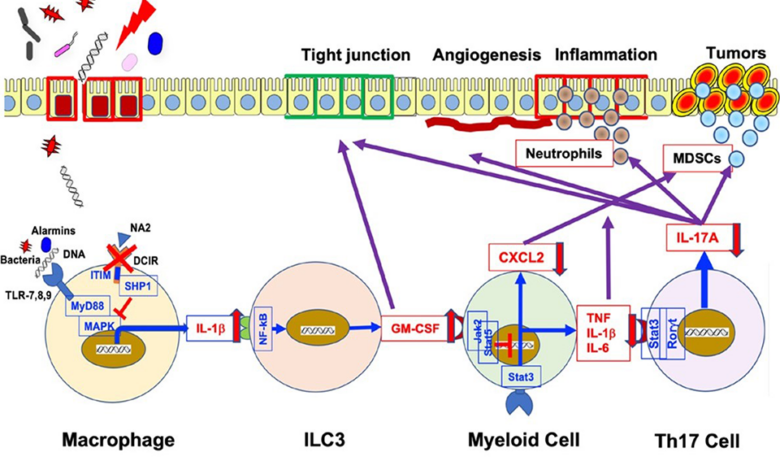
Colorectal tumor is a common side effect of chronic inflammatory bowel disease. In a new study, researchers from Japan and China demonstrate that mouse models lacking the dendritic cell immune receptor (DCIR) protein are resistant to dextran sodium-induced colon tumors. caused by sulfate and azoxymethane. Furthermore, they identified an antibody that reduces colitis severity and colon tumor growth, highlighting the potential of DCIR as a therapeutic target.

Image Caption: A new study by researchers from Japan and China has revealed that inhibiting DCIR, a C-type lectin receptor protein responsible for maintaining homeostasis in the immune system and bone, reduces the severity of DSS colitis symptoms and suppresses the growth of colorectal tumors. These findings may pave the way for DCIR-targeted therapeutic strategies for the treatment of colorectal tumors. Image credits: Suppression of colitis and colorectal tumors through inhibition of DCIR.
Inflammatory bowel disease (IBD) is an umbrella term for two diseases, Crohn’s disease and ulcerative colitis, characterized by prolonged inflammation of the digestive tract. This condition often leads to the development of colorectal tumors. Therefore, understanding the pathogenesis of IBD is important to minimize the incidence of colon tumors.
It turns out that innate immune receptors, particularly those expressed in the gut, such as C-type lectin receptors (CLRs), are responsible for the development of IBD. However, the CLR also plays an important role in regulating the gut microbiota and protecting against pathogens. Therefore, balance is required to maintain intestinal homeostasis.
The dendritic cell immune receptor (DCIR) is one of the CLRs responsible for maintaining the homeostasis of the immune and skeletal systems. Previous studies have suggested that DCIR negatively regulates both innate and acquired immune responses. Thus, blocking DCIR could enhance immunity against colon tumours. However, its role in intestinal immunity remains unclear.
Against this backdrop, a research team led by Professor Yoichiro Iwakura of Tokyo University of Science (TUS) in Japan has now shed some light on this issue. In their study, was Published online in international journals Mobile reportingteam studied the development of inflammatory bowel disease and colon tumors in DCIR-deficient mouse models.
To this end, the team gave the mice water containing dextran sodium sulfate (DSS), a synthetic polysaccharide sulfate, and azoxymethane (AOM), a neurotoxic chemical, to induce tumors. colon is similar to that observed in people with IBD.
To their surprise, they found that the DCIR-deficient mice showed reduced colitis severity and AOM-DSS-induced colorectal tumor growth. Furthermore, compared with wild-type (control) mice, DCIR-deficient mice showed lower body weight loss as well as decreased proinflammatory cell infiltration in the colon.
What do these observations imply? Professor Iwakura explained, “Our findings point to the fact that carcinogenesis and intestinal inflammation are facilitated by DCIR signaling, which suggests that blocking DCIR may prevent ulcerative colitis and cancer. colon.”
Demonstrating this possibility, the study also revealed that using an antibody called “anti-NA2” against asialo-biantennary-N-glycans (NA2), a ligand (binding molecule) with DCIR , relieves symptoms of DSS colitis and suppresses the growth of colorectal tumors. .
The researchers were delighted by these findings. Talking about the practical applications of their research, Professor Iwakura said, “Our results suggest that therapeutics targeting DCIR and its ligands can be used to effectively treat autoimmune diseases, IBD, and cancer, which are traditionally very common diseases. difficult to treat.”
Certainly, this study could open the door to new therapeutic strategies for the treatment of colorectal tumors, improving not only the lives of IBD patients but also our understanding of the underlying mechanisms. pathogenesis of human diseases.
Source: Tokyo University of Science




