Plug-and-Play Organ-on-a-chip can be customized for the patient
Designed tissues has become an important component for disease modeling and for testing drug efficacy and safety in human contexts. A major challenge for researchers is how to model bodily functions and systemic diseases with engineered tissues that can communicate physiologically – just as they do in the body.
However, it is essential to provide each genetically engineered tissue with its own environment so that specific tissue phenotypes can be maintained for weeks to months, as required by biological and medical studies. born.
Complicating the challenge is the need to link the modules together to facilitate their physiological communication, which is necessary for the modeling conditions involved. access to more than one organ system, without sacrificing the individually engineered tissue environment.

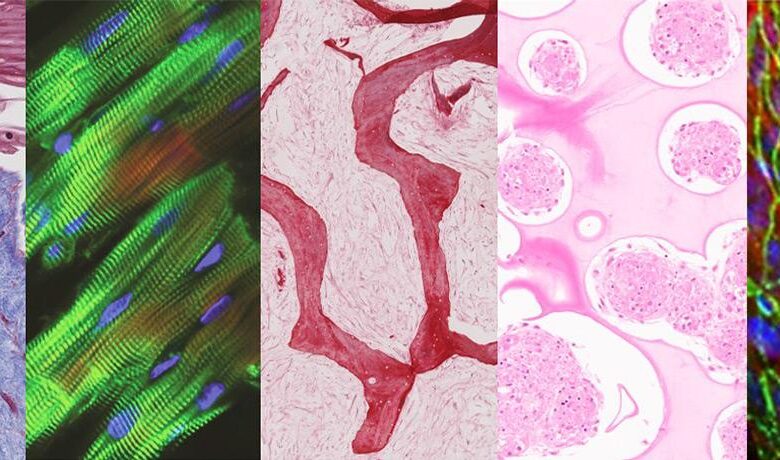
Tissue cultured in a multi-organ chip (left to right: skin, heart, bone, liver and endothelial barrier) maintains tissue-specific structure and function after being linked by vascular flow . Image credit: Kacey Ronaldson-Bouchard / Columbia Engineering
Novel plug-and-play multi-organ chip, customized for the patient
So far, no one has met both of these conditions. Today, a group of researchers from Columbia Engineering and Columbia University Irving Medical Center report that they have developed a model of human physiology in the form of a multi-organ chip that includes the human heart, bone, liver, and skin that has been engineered to be linked by vascular flow to cells. circulating immune cells, to allow resynthesis of the functions of interdependent organs.
The researchers have essentially created a plug-and-play multi-organ chip, about the size of a microscope slide, that can be customized for the patient. Because disease progression and response to treatment vary widely from person to person, such a chip will eventually allow personalized therapy optimization for each patient. The learn is the cover story of the issue Natural Biomedical Engineering.
“This is a huge achievement for us — we spent ten years doing hundreds of tests, discovering countless great ideas, and building many prototypes, and in the end we developed this platform to successfully capture the biology of organ interactions in said project leader Gordana Vunjak-NovakovicUniversity Professor and Mikati Foundation Professor of Biomedical engineeringMedical Science and Dental Medicine.
Inspired by the human body
Inspired by how the human body works, the team built a human tissue chip system in which they linked adult heart, liver, bone and skin modules by circulating blood vessels. , allowing interdependent organs to communicate just like in the human body.
The researchers chose these tissues because they are of embryonic origin, have distinctly different structural and functional properties, and are adversely affected by cancer drugs.
“Providing communication between tissues while preserving their individual phenotypes is one thing,” said Kacey Ronaldson-Bouchard, lead author of the study and an associate research scientist at Vunjak-Novakovic’s. big challenge. Stem Cell and Tissue Engineering Laboratory.
“Because we focus on using models taken from patients, we had to individually mature each tissue so it behaves in a way that mimics the responses you would see in a patient and we didn’t want to. sacrificing this advanced functionality when connecting multiple tissues. In the body, each organ maintains its own environment and interacts with other organs by means of a vascular current carrying circulating cells and biologically active factors. So we chose to connect tissues by means of vascular circulation, while preserving the individual tissue niches needed to maintain its biological fidelity, mimicking the way our organs connected in the body. ”

The new multi-organ chip is about the size of a microscope slide and allows the culture of up to four engineered human tissues, whose location and quantity can be tailored to the question asked. out. These tissues are interconnected by vascular flow, but the presence of a selectively permeable endothelial barrier maintains their tissue-specific niche. Image credit: Kacey Ronaldson-Bouchard / Columbia Engineering
Optimized modular modules can be maintained for more than a month
The team created modular modules, each in its own optimal environment, and separated them from the general vascular flow by a selectively permeable endothelial barrier. Individual tissue environments can communicate across endothelial barriers and through the vascular circulation. The researchers also introduced macrophage-producing monocytes to the circulatory system, because of their important role in directing tissue responses to injury, disease, and regulatory outcomes. treat.
All tissues were obtained from the same induced human pluripotent stem cell (iPSC) line, obtained from a small blood sample, to demonstrate capacity for individual, patient-specific studies . And, to demonstrate that the model could be used for long-term studies, the team maintained the tissues, which had been cultured and matured for four to six weeks, an additional four weeks, after they linked together by perfusion.
Using models to study anti-cancer drugs
The researchers also wanted to demonstrate how this model could be used for studies of an important systemic condition in a human context, and have chosen to examine the side effects of anticancer drugs. . They studied the effects of doxorubicin – a widely used anti-cancer drug – on the heart, liver, bones, skin and blood vessels. They show that the measured effects aggregate reports from clinical studies of cancer therapy using the same drug.
The team developed in parallel a new computational model of the multiorgan chip to mathematically simulate drug absorption, distribution, metabolism and excretion. This model accurately predicted the conversion of doxorubicin to doxorubicinol and its diffusion into the chip. The combination of the multi-organ chip with the computational approach in future studies of the pharmacokinetics and pharmacodynamics of other drugs provides an improved basis for preclinical and preclinical extrapolation, with improvements in the drug development process.
“In doing that, we were also able to identify some early molecular markers of cardiotoxicity, the main side effect that limits the drug’s widespread use. Most notably, the multi-organ chip accurately predicted cardiotoxicity and cardiomyopathy often requiring clinicians to reduce therapeutic doses of doxorubicin or even discontinue therapy,” said Vunjak-Novakovic.

For their study, the researchers cultured liver, heart, bone, and skin, connected by vascular flow, for four weeks. These tissues can be generated from a single human pluripotent stem cell, creating a patient-specific chip, an excellent model for individual studies of human disease and testing. drug test. Image credit: Keith Yeager / Columbia Engineering
Cooperation throughout the university
The development of the multi-organ chip started from a background with the heart, liver and vascular system, nicknamed the HeLiVa communication.
As always with Vunjak-Novakovic biomedical research, collaboration is crucial to getting the job done. These include the collective talent of her lab, Andrea Califano and his systems biology team (Columbia University), Christopher S. Chen (Boston University) and Karen K. Hirschi (University of Virginia) with their expertise in vascular engineering and biology, Angela M Christiano and her skin research team (Columbia University), Rajesh K. Soni of Proteomics Core at Columbia University, and computational modeling assistance team at CFD Research Corporation.
Countless applications, all in patient-specific contexts
The team is now using variations of this chip to study, all in patient-specific contexts: breast cancer metastasis; metastatic prostate cancer; leukemia; the effect of radiation on human tissues; effects of SARS-CoV-2 on the heart, lungs and blood vessels; the effect of ischemia on the heart and brain; and the safety and effectiveness of the drug. The team is also developing a user-friendly standardized chip for both academic and clinical labs, to help leverage its full potential in advancing biological and medical research.
Vunjak-Novakovic added, “After ten years of research on organs on a chip, we still find it amazing that we can model the physiology of a patient by connecting millimeter-sized tissues — muscles The heart is beating, the liver is metabolizing and the skin is functioning, and bones are grown from the patient’s cells. We are excited about the potential of this approach. It is uniquely designed for studies of systemic conditions associated with injury or disease and will allow us to maintain the biological properties of engineered human tissues with the communication of they. One patient at a time, from inflammation to cancer! ”
The source: Columbia University