Frequency Therapeutics redesigns study after disappointing Phase 2 results, debuts new hearing loss and MS drugs – TechCrunch

In Frequency Therapeutics’ quick lifetime as an organization, it’s had its ups and downs. On Tuesday, throughout an R&D occasion day, the corporate unleashed a slew of bulletins that add context to the event of a flagship listening to loss drug, and suggest some instructions.
Based in 2015, Frequency Therapeutics has centered on a regenerative medication method to listening to loss. The method is centered on revamping progenitor cells that finally change into key sound-conducting hair cells within the cochlea. The irreversible disappearance or damage of these hair cells contributes to sensorineural listening to loss – the commonest kind of listening to loss.
On Tuesday, the corporate made a number of bulletins relating to its FX-322 – it’s drug candidate for listening to loss. Frequency highlighted pooled knowledge from early trials suggesting the product has result in medical advantages, and urged {that a} Part 2 research’s discouraging outcomes have been on account of poor research design. Then, it unveiled a brand new listening to loss product, and a a number of sclerosis drug program.
Reforming FX-322 research design
Most circumstances of sensorineural listening to loss are handled with both cochlear implants, or with listening to aids. Individuals who expertise sudden listening to loss would possibly try steroid treatments, but there are not any medication authorised to deal with or reverse sensorineural listening to loss.
FX-322 acquired off to a superb begin. One Phase 1b study on 15 individuals who acquired the FX-322 drug and eight individuals who acquired placebo injections didn’t detect any adversarial uncomfortable side effects. 4 individuals who acquired the drug noticed clinically significant enchancment of their capability to listen to particular phrases.
A aspect word: Frequency measures success of its drug primarily based on “speech notion” quite than a capability to easily hear sounds. Other trials on cochlear implants have used this endpoint, and Chief Scientific Officer Chris Free, says that the corporate has “alignment” with the FDA on the utility of measuring listening to medication this manner.
Over time, these outcomes have held up, stated Carl LeBel, Frequency’s chief growth officer. Unpublished sturdiness knowledge from 5 contributors which were adopted over one to 2 years discovered that three topics are nonetheless seeing statistically vital enhancements, he instructed Tech Crunch.
“That, to us, urged some topics are in a position to keep the profit. That profit might final a yr, it might final two years. We simply have to watch extra sufferers that present this enchancment, however it actually suggests a illness modifying profit,” stated LeBel.
However the excellent news on FX-322 has been tempered. A Part 2a medical trial urged the machine had not proven any enchancment in listening to loss in comparison with placebo.
That research was carried out on 95 contributors, half of whom acquired 4 injections of the drug, whereas the opposite half acquired placebos. There have been restricted enhancements in each teams and the corporate reported there was “no discernible benefit” of the drug in that trial.
Shares of Frequency dropped from $36 to $7 the day the information was introduced, and have remained well below earlier highs since. In response, some shareholders have filed a class-action lawsuit claiming management misrepresented FX-322 in earnings calls, press releases, SEC filings and shows earlier than March 23, 2021.
At this level, firm management argues that this research was tainted by bias — as a result of sufferers might have underreported their very own listening to capability to achieve entry to the trial, stated LeBel. And the research didn’t adequately management for that chance.
“As sufferers have been coming in to the research, there have been inconsistencies between their historic speech notion scores and the scores that they have been coming into on the baseline go to of the research,” LeBel instructed TechCrunch.
Jason Glashow, Frequency’s senior vice chairman of company affairs, clarified that the corporate sees this as a “design challenge” with the trial.
“Whereas there may be bias within the research it’s not the fault of people that participated,” he instructed TechCrunch.
Throughout the R&D day, Frequency reported pooled knowledge from three of the Part I research to make the case that FX-322 was displaying a sample of response, and that the Part 2 research was an outlier.
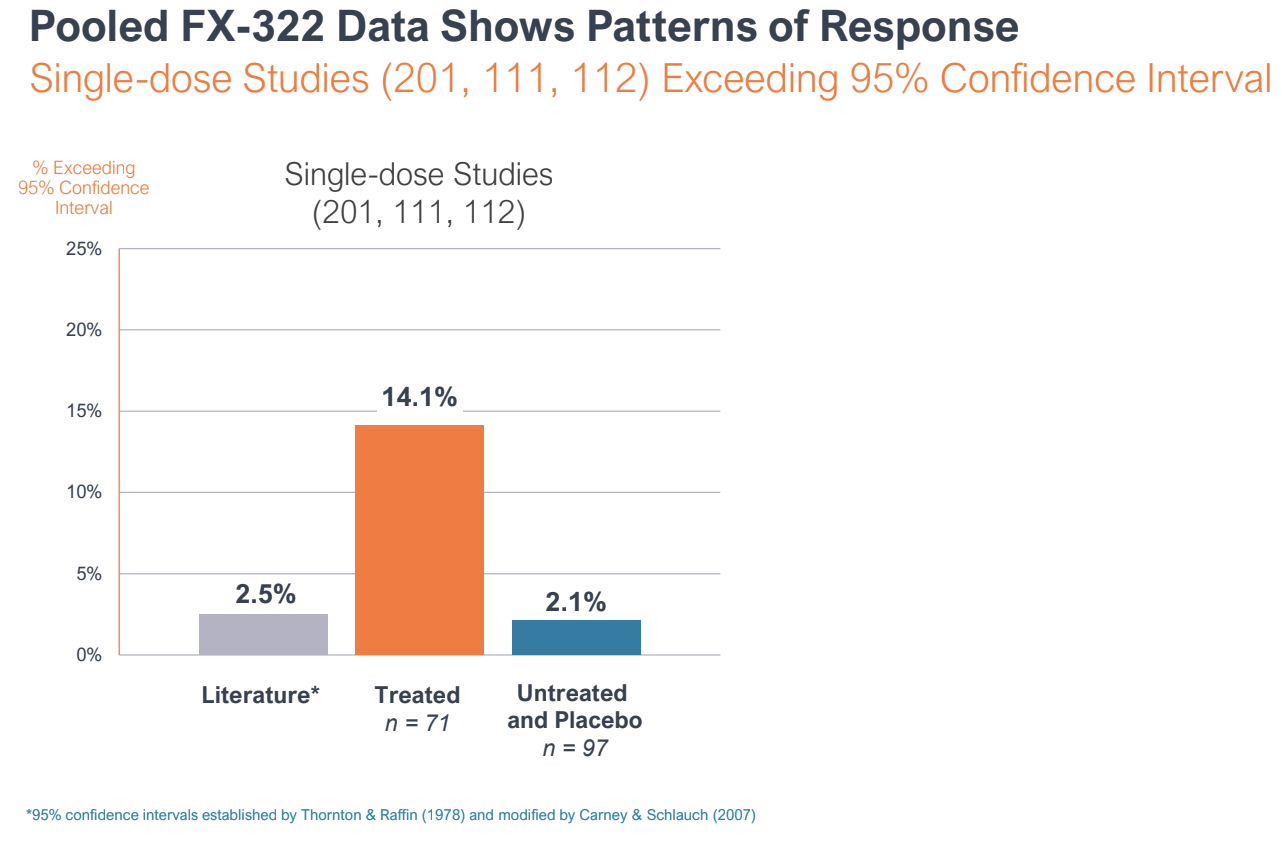
Pooled knowledge from three of Frequency Therapeutics trials on FX-322 confirmed a sample of response. A Part 2 medical trial confirmed no impact in comparison with placebo, however management argues the research suffered from bias and poor design.
It’s unclear how this information might or might not affect the continuing authorized proceedings. However the expertise has knowledgeable the development of future research on the FX-322 drug.
Frequency has already introduced the start of a brand new Part 2b medical trial on FX-322. This research on 124 contributors included a one month “lead in” part to watch contributors’ listening to earlier than baseline listening to is measured. The primary affected person was dosed in October 2021.
It is going to additionally slender the concentrate on what kind of listening to loss the corporate is concentrating on: these topics may have identified noise-induced or sudden sensorimotor listening to loss. It’s a tremendous distinction, however barely adjustments the parameters of what varieties of listening to loss the remedy targets. (Nonetheless, noise-induced listening to loss impacts between 10 and 40 million people per year, per CDC estimates).
A brand new drug candidate and MS program
Except for FX-322, Frequency is, for the primary time, trying to wager on greater than only one product to ascertain itself. The corporate additionally has plans to check a brand new product, referred to as FX-345, a stronger model of the small molecule in FX-322. That efficiency, stated Free, offers this product the power to penetrate deeper into the cochlea.
The corporate will likely be pursuing an Investigational New Drug Utility IND (IND) in Q2 of subsequent yr.
Frequency can also be within the technique of growing a a number of sclerosis drug referred to as FX-162 – one other step towards one of many firm’s earlier articulated objectives: to focus extra broadly on regenerative medication.
In keeping with a presentation seen by TechCrunch, the corporate does have preliminary knowledge from mouse research suggesting the drug has been in a position to assist drive oligodendrocyte manufacturing. These cells produce the fatty sheath that’s degraded in sufferers with a number of sclerosis.
Nonetheless, the corporate wouldn’t disclose a timeline for upcoming research.
For now, the main target stays on FX-322, and on the newly designed trials that may present solutions to some excellent questions.




