FDA Approves lecanemab for Biogen, Eisai

An MRI image shows an area of the brain of an Alzheimer’s patient.
beautiful pictures
The Food and Drug Administration on Friday granted expedited approval for the Alzheimer’s drug lecanemab, the second treatment from biological and Japanese partner Eisai will get the green light soon in less than two years.
The FDA approval comes after clinical trial results released in November showed that lecanemab somewhat slowed cognitive decline in people with mild impairments from Alzheimer’s disease, but the treatment There is also a risk of brain swelling and bleeding.
Eisai, who led the development of lecanemab, is valuing the treatment at $26,500 each year in the US It will be sold under the name Leqembi.
The FDA can speed up the approval of a drug to get it to market quickly if it is expected to help patients with more serious illnesses than what’s currently available. Biogen and Eisai applied for expedited approval in July.
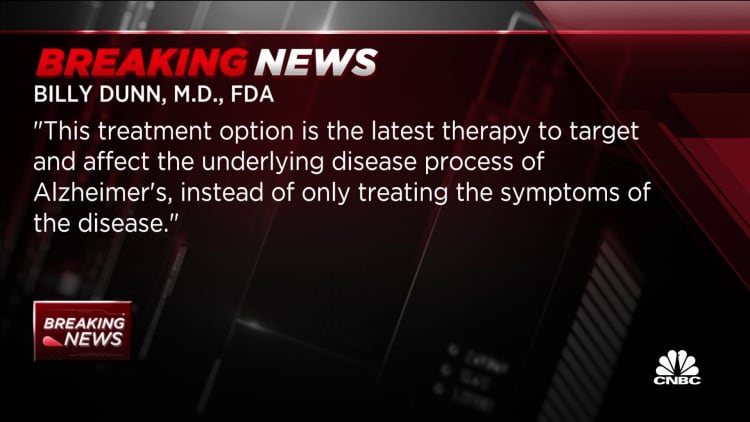
“Alzheimer’s disease impairs the lives of people with the disease and has a devastating impact on their loved ones,” said Dr. Billy Dunn, director of the FDA’s division of neuroscience. “This treatment option is the newest therapy that targets and affects the underlying disease process of Alzheimer’s disease, rather than just treating the symptoms of the disease.”
More than 6.5 million people in the US have Alzheimer’s disease. The irreversible disease destroys memory, thinking skills and ultimately the ability to perform simple tasks.
The decision on lecanameb comes after Congress released a scathing report last week on how the FDA handled the controversial approval of another Alzheimer’s drug being developed by Biogen and Eisai, which has The name is Aduhelm. Approval of that treatment in 2021, which experts say shows no clear clinical benefit, is “fraught with irregularities,” according to the report.
The congressional report said “The FDA must act quickly to ensure that its processes for reviewing future Alzheimer’s treatments do not lead to the same doubts about the truthfulness of the review.” by the FDA.”
Just to slow down the disease
Lecanemab is a monoclonal antibody that targets a protein called amyloid that builds up on the brain in people with Alzheimer’s disease. Antibodies are administered intravenously every two weeks at a dose determined by the patient’s body weight of 10 milligrams per kilogram.
According to a statement from the agency, the FDA approved lecanemab based on the reduction in amyloid plaque observed in clinical trial participants who received the treatment. Participants in the untreated, placebo arm, had no reduction in amyloid plaques.
Clinical trial results, Published in the New England Journal of Medicine, found that cognitive decline was 27% slower over 18 months in those taking lecanemab compared with those not receiving the treatment. The study was funded by Biogen and Eisai.
Cognitive decline is measured using a system known as the clinical dementia assessment, which is an 18-point scale with a higher score indicating a greater degree of impairment. It measures cognitive functions such as memory, judgment, and problem solving.
Alzheimer’s disease progression averaged 1.21 points in the lecanemab group compared with 1.66 points in the no-treatment group, a modest difference of 0.45 points.
Nearly 1,800 people aged 50 to 90 with early-stage Alzheimer’s participated in the trial, about half of whom received lecanemab and half of them did not.
safety concerns
Although lecanemab may slow cognitive decline somewhat, the treatment also carries risks.
Nearly 13% of those taking lecanemab developed brain swelling compared with about 2% in the untreated group. However, most of these cases are mild to moderate in severity, cause no symptoms, and usually resolve within four months.
About 3% of patients taking lecanemab experienced more severe brain swelling with symptoms including headache, visual disturbances, and confusion.
About 17% of those taking lecanemab experienced a brain bleed, compared with 9% in the no-treatment group. The most common symptom associated with bleeding is dizziness.
Overall, 14% of those taking lecanemab experienced serious side effects in the clinical trial, compared with 11% of those who did not receive the treatment.
The study’s authors say longer clinical trials are needed to determine the efficacy and safety of lecanemab in patients with early-stage Alzheimer’s disease.
The FDA says prescribing information for lecanemab will include a warning about the risk of swelling and bleeding, commonly known as amyloid-associated imaging abnormalities.
According to one study, the death of a clinical trial participant in the Chicago area could also be related to lecanemab. The study letter was published in the New England Journal of Medicine this week.
The 65-year-old man had a stroke and was hospitalized four days after their third lecanemab infusion. A CT scan performed after a patient’s stroke revealed massive bleeding in the brain. Magnetic resonance imaging 81 days before the stroke showed no bleeding.
The patient also received a drug called t-PA, which is used to break up the blood clot that caused the stroke. But extensive brain bleeding would be an unusual complication with the drug alone, according to the doctors who wrote the research letter.
The researchers involved in the lecanemab clinical trial, in a response letter, argued that the clot-busting drug appeared to be the direct cause of the patient’s death, with symptoms first. appeared 8 minutes after they were given a clot-busting drug.




