A Potential Resource Crisis That Could Stifle Green Technology and Threaten Food Security as The World Decarbonizes – Watts Up With That?

HT/Alastair B
Mark Maslin, Livia Van Heerde, Simon Day
First published: 21 August 2022 | https://doi.org/10.1111/geoj.12475
Abstract
Sulfur in the form of sulfuric acid is a crucial part of our modern industrial society. It is required for the production of phosphorus fertiliser and manufacturing lightweight electric motors and high-performance lithium-ion batteries. Over 246 million tonnes of sulfuric acid are used annually. Rapid growth in the green economy and intensive agriculture could see demand increase to over 400 million tonnes by 2040. Today over 80% of the global sulfur supply comes from desulfurisation of fossil fuels to reduce emissions of sulfur dioxide (SO2) gas. Decarbonisation of the global economy to deal with climate change will greatly reduce the production of fossil fuels. This will create a shortfall in the annual supply of sulfuric acid of between 100 and 320 million tonnes by 2040, depending on how quickly decarbonisation occurs. Unless action is taken to reduce the need for sulfuric acid, a massive increase in environmentally damaging mining will be required to fill this resource demand.
Short Abstract
Sulfur in the form of sulfuric acid is a crucial part of our modern industrial society. It is required for the production of phosphorus fertiliser and manufacturing lightweight electric motors and high-performance lithium-ion batteries. Today over 80% of the global sulfur supply comes from desulfurisation of fossil fuels to reduce emissions of sulfur dioxide (SO2) gas. Decarbonisation of the global economy to deal with climate change will greatly reduce the production of fossil fuels. This will create a shortfall in the annual supply of sulfuric acid of between 100 and 320 million tonnes by 2040. Unless action is taken to reduce the need for sulfuric acid, a massive increase in environmentally damaging mining will be required to fill this resource demand.
1 INTRODUCTION
Sulfur, in the form of sulfuric acid, is a vital ingredient for many industries. The acid is used in producing phosphorus fertiliser (Cordell et al., 2009), lightweight electric motors, and lithium-ion batteries (Childers et al., 2011; IEA, 2021a; Ober, 2002). It is vital for extracting metals from ores and manufacturing polymers. Yet a problem looms, which is largely unnoticed. As green technology booms and agriculture intensifies, global demand for sulfuric acid is set to rise significantly by 2040. At the same time, the major present-day source of sulfur for these industries will rapidly diminish with the decline in production of oil and gas (BP, 2021; IEA, 2021a).
More than 80% of the sulfur used industrially (USGS, 2022) comes from oil and natural gas, which typically contain 1–3 weight percent sulfur (Speight, 2019). The element is removed during refining to enable use of noble metal catalysts in further refining downstream and, most significantly, to reduce combustion emissions of sulfur dioxide (SO2) gas, a major cause of air pollution and acid rain (Grennfelt et al., 2020). As the world decarbonises over the next three decades (IPCC, 2018, 2022), the supplies of sulfur will drop, just when the material is needed most (IEA, 2021a). Prices of sulfuric acid will rise, which could increase the cost of food, especially if sulfuric acid-using green tech industries outbid fertiliser producers. As ever, developing countries will be hit hardest.
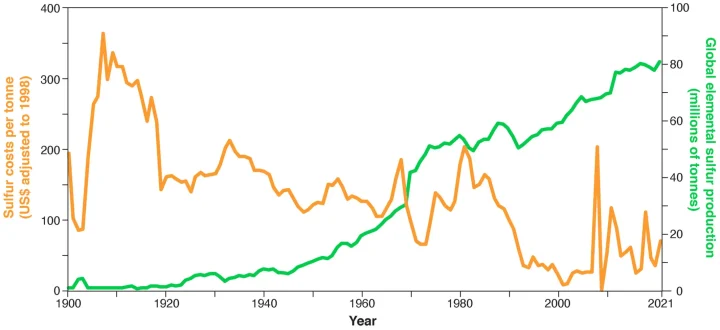
The sulfur crisis is not a new one. In the 1950s there were concerns about supplies of industrial sulfur (AC, 1951), which was ultimately solved as an unintended consequence of the oil refining industry having to remove organosulfur compounds which make platinum-based catalysts in hydrocarbon cracking reactors ineffective and then later on the regulation requiring the desulphurisation of fossil fuels. The question is why has not the latest threat to sulfur supplies been recognised? Partly this is because sulfur seems plentiful, available, and cheap today. There are almost limitless theoretical reserves of sulfur in geological deposits (USGS, 2022), although these are mainly sulfates whose present-day utility is limited. Furthermore, sulfur is also seen as a hazardous waste product (or a ‘fatal product’) in the fossil fuel industry, from whose perspective the sulfur-consuming, sulfuric acid-using industries can be seen as providing a waste disposal service, in which the sulfur is exchanged for the provision of that service. Thus, sulfur production is in effect highly subsidised, producing sulfur that is provided at very low direct monetary cost to the sulfur-using industries, and in volumes that may even occasionally exceed the world’s current needs (Wagenfeld et al., 2019). Prices of sulfur have remained at less than $40 per tonne (inflation adjusted) for the last 40 years (Ober, 2002), except when recessions and/or oil conflicts have caused supply–demand imbalances and temporary sulfur shortages (Figure 1). In such situations the price can spike to over $200 per tonne, as in the post-2008 recession, but its average price over that period was still significantly below the inflation-adjusted cost of alternative methods of sulfur production as a primary product, such as the Frasch process that dominated the world sulfur supply between the 1920s and 1970s (Figure 1; Ober, 2002). Furthermore, the hydrocarbon desulfurisation process produces the raw element ‘sulfur’ in powder or chips that can be transported and converted to sulfuric acid at major points of use, minimising the cost of transport to the user. Low costs are vital for any material used in bulk. It can take 250 tonnes of sulfur to extract 1 tonne of cobalt metal from lateritic ore (Dunn et al., 2015; Tagliaferri et al., 2016).


Prior to the dominance of sulfur production by desulfurisation of fossil fuels, sulfur was also produced by direct mining of elemental sulfur or as a byproduct from mining of copper and other sulfide minerals, which by contrast is dirty and expensive. Many sulfide minerals also contain heavy metals like mercury, arsenic, and thallium, which are toxic. Furthermore, roasting sulfide minerals produces sulfur dioxide gas that has to be converted immediately to concentrated sulfuric acid, which is more expensive and dangerous to store and transport than elemental sulfur. Elemental sulfur occurring around salt domes can be extracted in the Frasch process by injecting superheated steam to melt the sulfur and propel it to the surface, but this demands thermal energy that is most easily (and cheaply, prior to the 1970s) supplied by burning fossil fuels and generates large volumes of wastewater contaminated with sulfur and hydrogen sulfide. Thus, wide use of the Frasch process has energy and environmental costs that were acceptable prior to the 1980s, but should be unacceptable now.
2 GROWING SUPPLY–DEMAND GAP
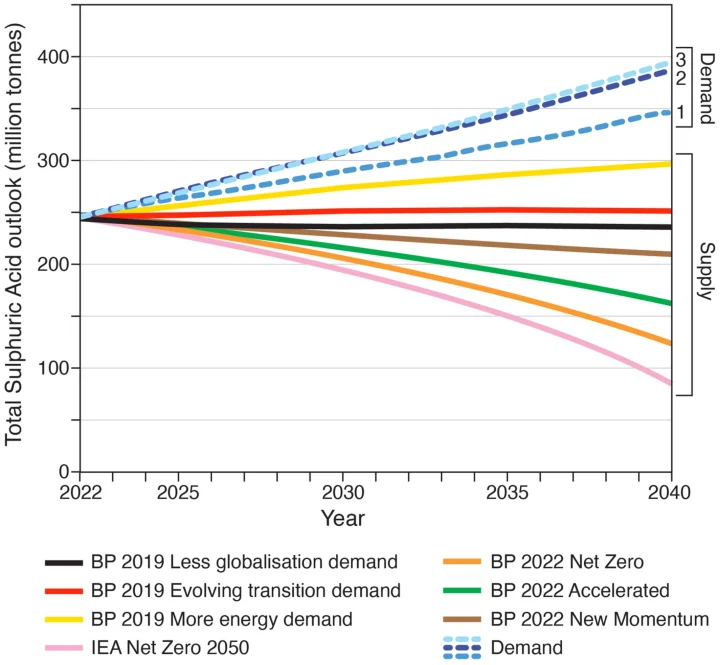
To forecast sulfuric acid demand, three scenarios were modelled for 2021 to 2040 based on three different sources (shown in Figure 2). The scenarios are based on historic and forecast sulfuric acid demand. Scenario 1 is based on an annual growth rate of 1.8%, calculated from the 2011–18 sulfuric acid demand which has been extended forward to 2040 (Essential Chemical Industry, 2016). Scenario 2 uses a larger published average annual growth rate of 2.3% annually, calculated from the 2015 to 2021 sulfuric acid demand (Statista, 2020). Scenario 3 uses a slightly higher estimated growth rate of 2.4% (Shah, 2019), based on 2018 sulfuric acid demand and predictions to 2027. Other more extreme scenarios based on very rapid expansion of electric vehicles and rare metal batteries (IEA, 2021a, 2021b; McKinsey and Company, 2018) were discounted until there is more evidence of this increase. However, this additional factor of potential increase in demand for sulfuric acid from these new industries suggests that our demand curves are conservative estimates and that future demand could be much higher.
Sulfuric acid supply projections, also shown in Figure 2, are based on sulfur recovery from oil use projections (IEA, 2021b; BP, 2019, 2022). The global USGS (2022) sulfur supply data for 2021 were used as a baseline for the calculations (~81 million tonnes equivalent to ~246 million tonnes of sulfuric acid). Sulfur recovered from natural gas and other sources was assumed to be constant because there is considerable uncertainty over future production, particularly over the next decade. First, this is because the pandemic caused the demand for natural gas to drop by 4% in 2020 (BP, 2020; Shiryaevskaya & Dezem, 2020), but this is predicted to rise by 1.5% by 2025, after which it could vary by ±25% of the 2021 level by 2040 (BP, 2022). Second, the Russian invasion of Ukraine has accelerated EU plans to transition away from natural gas, with new plans to triple the planned renewable energy infrastructure by 2030 (Hockenos, 2022). Third, the IEA (2021b) suggests a 75% drop in natural gas is required by 2040 to be consistent with the 1.5°C climate pathway – but there is currently no evidence that this will occur.
Six future oil demand scenarios were chosen to illustrate the huge range of possibilities in the next few decades. Three BP (2019) scenarios were chosen to illustrate possible changing supply: more fossil fuel energy demand and less globalisation demand. Three BP (2022) scenarios trying to model the energy transition away from fossil fuels, including: new momentum, accelerated, and the net zero pathway. These are compared to the IEA (2021b) 1.5°C decarbonisation pathway. Depending on how rapidly the world decarbonises and the amount of negative carbon emissions used to offset fossil fuel use, there could be a shortfall in sulfuric acid of between 100 and 320 million tonnes. That is a shortfall of between 40% and 130% of current production by 2040. Hence, rapid reductions in demand and/or massive increases mining are needed in the coming decades.
3 DIRTY MINING
The rapid expansion of green technologies is predicted to significantly increase the need to mine minerals and metals (Herrington, 2021). Cobalt demand could increase by 460%, nickel by 99%, and neodymium by 37% by 2050 (Herrington, 2021), all of which currently use large amounts of sulfuric acid in their extraction. The USGS (2022) estimates there is almost limitless supply of elemental sulfur and sulfate minerals in evaporites, volcanic deposits, gypsum, and anhydrite, but new industries will be required to reduce sulfates to sulfur in order to exploit most of these resources. China already subsidises carbothermal reduction of gypsum to produce sulfuric acid directly in integrated industrial eco-parks with co-located acid-using industries (Zhang et al., 2015), but at the environmental cost of large CO2 emissions.
More immediately, the sulfur shortfall could be offset by expanding mining of sulfides and elemental sulfur, but at large environmental costs. This could include both conventional mining of sulfur deposits and the Frasch mining process that extracts elemental sulfur from salt domes or bedded evaporite deposits by injecting super-heated water into the deposits (Ober, 2002). This will create environmental problems, such as air, soil, and water pollution, and human rights issues associated with intensive mining (Martin & Iles, 2020). Sulfide mining operations also have their own issues. Of particular concern is mining wastes containing sulfide minerals (Chopard et al., 2019) that can acidify local surface and ground waters and increase the levels of numerous toxic elements (As, Bi, Co, Hg, Ni, Tl, Sb, Se, etc.). At the same time, in South China, direct generation of sulfuric acid continues not only by roasting of copper and other sulfide ores to produce valuable metals with sulfuric acid as a byproduct (Han et al., 2016) but also by roasting pyrite to produce sulfur dioxide and hence sulfuric acid as the primary product, at the cost of substantial heavy metal and especially thallium pollution of the region (Liu et al., 2016).
Research is urgently needed to develop low-cost, low environmental impact methods of extracting large quantities of elemental sulfur from the very abundant deposits of sulfate minerals such as gypsum and anhydrite. The international community needs to consider supporting and regulating sulfur mining to minimise the impacts and also to avoid cheap unethical production from distorting the market. A particular problem is the need to manage the decline of sulfur production from fossil fuel desulfurisation, so that temporary increases in supplies of waste sulfur from this source do not collapse the market price of sulfur and so undermine the longer-term development of sulfur production from new low environmental impact sources.
4 INCREASE RECYCLING
Alternatively, the demand for sulfur could be reduced, both from the presently dominant fertiliser industry and from new users consuming increasing amounts of sulfuric acid as part of the transition to post-fossil fuel economies. In terms of phosphorus fertilisers, recycling sewage and other waste (Cordell et al., 2011) is a viable alternative to sulfuric acid processing of phosphate rock (Withers et al., 2015). In sewage treatment works, phosphate can be precipitated out as struvite (Mg[NH4]PO4), a good combined N and P fertiliser, although the Mg can be problematic for some soils and crops. Research is required as struvite-saturated solutions can cause damaging deposits in pumps, machinery, and pipes. Recycling of phosphate from sewage would, in the longer term, also help to address the “peak phosphorus” problem due to the predicted future scarcity of phosphate rock (McGill, 2012). It may also ameliorate the issue of phosphorus eutrophication (Chislock et al., 2013) in many freshwater and coastal areas and make a major contribution to the Sustainability Development Goal 6.3.
Demand for sulfuric acid from new industries could be reduced somewhat by increasing the recycling of lithium batteries (Harper et al., 2019; but it should be noted that recycling will have little impact in the period to 2040 and beyond in which the production of new batteries will greatly increase from a low initial base), or by using lower energy capacity/weight ratio batteries such as the Li-FePO4 battery (Durmus et al., 2020). These require less sulfur for their production compared to the highest capacity Li-ion batteries that at present use Ni-Co cathode chemistries. The next research step is to invent new more powerful batteries and motors that are less reliant on Ni, Co, and rare earth elements. This would boost energy storage and efficiency, accelerating decarbonisation and reducing the need for sulfuric acid. Another increment of reduction in sulfur demand might also come from alternatives to vulcanised rubber in vehicle tires, to offset the greater demand for this material resulting from the increased weights of battery electric as compared to internal combustion engine vehicles.
More speculative alternatives to sulfur production from fossil fuels could include developing techniques to recycle sulfur from the sulfate salts produced in sulfuric acid using processes, through its different oxidation states using sulfur-cycle bacteria. Bacteria are already used at small scale in some of the steps involved, for example in the Shell-Paques process for converting hydrogen sulfide in natural gas to sulfur, but industrial-scale bacterial reduction of sulfates to produce hydrogen sulfide gas has not yet been implemented (Cline et al., 2003; De Crisci et al., 2019; Muyzer & Stams, 2008) – in part due to the extremely hazardous nature of the product. It may also be possible to replace sulfuric acid with different acids, such as nitric acid, in those industrial processes where this can be done without creating toxic, environmentally damaging, and/or radioactive problems (Schnug & Lottermoser, 2013). For example, nitric acid is a potential substitute for processing Ni-Co laterites, but not for processing phosphate rock because it creates waste water containing radioactive and highly soluble uranyl nitrate (Ma et al., 2015). However, the production of nitric acid is itself expensive, presently depends on supplies of ammonia derived from natural gas, and would require expensive changes to industrial processes presently using sulfuric acid.
5 CONCLUSIONS: THE NEXT STEPS
Decarbonising the global economy is essential if the impacts of climate change are to be restricted (IPCC, 2018, 2022). But it can have unintended consequences, which need to be acknowledged and solved – such as the potential sulfuric acid crisis. What makes the sulfur issue so difficult to deal with is that there is currently an extremely cheap plentiful supply. Critics could argue that it does not make economic sense to invest in alternative production or reduce the need until that supply decreases, particularly as we currently cannot accurately predict how quickly the subsidised supply of sulfur will decrease because decarbonisation of the global economy is only just starting. But our concern is that the dwindling supply could lead to a transition period when green tech outbids the fertiliser industry for the limited more expensive sulfur supply, creating an issue with food production, particularly in developing countries. By recognising the sulfur crisis now, we can develop national and international policies to manage future sulfur demand, increase resource recycling, and develop alternative cheap supplies which have minimal environmental and social impact.
5 Box: The many uses of sulfuric acid through time
Sulfur as an element has been used since ancient times in China, Egypt, Greece, and India (Kutney, 2013). Referred to as “brimstone”, it was used for medicine, fabric bleaching, and later as a key component of Chinese black powder or gun powder (Kutney, 2013), which changed the course of world history (Lewis and Maslin, 2018). The development of mining and refinement of sulfur led to more uses of the element, including furniture inlays, buildings, concrete, and fertiliser production (Thomson, 1995). Later in the 19th century, its consumption by the Great Powers (UK, USA, France, Germany) became a key index of industrial and military strength because sulfuric acid was used to produce nitric acid from nitrate minerals, to create nitrate organic compounds for advanced explosives and ammunition propellants. The connection between sulfur and economic and military strength became still stronger in the first half of the 20th century as mechanisation of road transport and mobile warfare were enabled by sulfur vulcanisation of rubber to produce durable vehicle tyres. Sulfur today is used as raw material for the production of paper, soaps, detergents, and industrial organic chemicals (Ober, 2002). However, its greatest significance today lies not in applications in where it is part of the final product, but in technologies where sulfuric acid is a key process or industrial chemical, used to decompose and dissolve a very wide range of different materials (Ober, 2002). Sulfuric acid is used to produce cellulosic fibres such as rayon or nylon, synthetic rubbers, drugs, nitrogenous and phosphorus fertilisers, pesticides, explosives, storage batteries, and acids in particular hydrofluoric acid, which is critical to the aluminium production, nuclear fuel processing, and semiconductor industries (Cheremisina et al., 2019; Yara, 2020). Sulfuric acid is also essential in the extraction, processing, and refining of a range of ferrous and nonferrous metals, that are used extensively in the tech-industry (Cheremisina et al., 2019). Over the last 100 years the method of production of sulfur has changed, as has the unit cost. See Figure 3 for annotated explanations of these changes.



ACKNOWLEDGEMENTS
The authors would like to thank the reviewers and the editors for all their helpful and supportive comments. We would also like to thank Miles Irving and the UCL Geography Drawing Office for assistance with the diagrams.
FUNDING INFORMATION
We would like to thank the Natural Environment Research Council London DTP (NE/L002485/1) for providing funding.
DATA AVAILABILITY STATEMENT
All the data used in this paper are publicly available and can be accessed through the references and websites cited in the main text and in the figure captions.
REFERENCES
- A.C. (1951) The world Sulphur shortage. The World Today, 7(9), 377– 383.Google Scholar
- BP. (2019) BP Energy Outlook 2019 edition The Energy Outlook explores the forces shaping the global energy transition out to 2040 and the key uncertainties surrounding that. BP Energy Outlook 2019, 22– 24, 72–76, 144–118.Google Scholar
- BP. (2020) Energy Outlook, Fuels, Gas. Available from: https://www.bp.com/en/global/corporate/energy-economics/energy-outlook/demand-by-fuel/natural-gas.html. Accessed August 2022.Google Scholar
- BP. (2021) Statistical Review of World Energy 2021, 70th edition Available from: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html. Accessed August 2022.Google Scholar
- BP. (2022) BP Energy Outlook 2022 edition Available from: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2022.pdf. Accessed August 2022.Google Scholar
- Cheremisina, O.V., Sergeev, V.V., Fedorov, A.T. & Il’ina, A.P. (2019) Rare earth metal extraction from apatite ores. Metallurgist, 63(3–4), 300– 307. Available from: https://doi.org/10.1007/s11015-019-00824-9CrossrefCASWeb of Science®Google Scholar
- Childers, D.L., Corman, J., Edwards, M. & Elser, J.J. (2011) Sustainability challenges of phosphorus and food: Solutions from closing the human phosphorus cycle. Bioscience, 61(2), 117– 124. Available from: https://doi.org/10.1525/bio.2011.61.2.6CrossrefWeb of Science®Google Scholar
- Chislock, M.F., Doster, E., Zitomer, R. & Wilson, A.E. (2013) Eutrophication: Causes, consequences, and controls in aquatic ecosystems. Nature, 4, 10.Google Scholar
- Chopard, A., Marion, P., Mermillod-Blondin, R., Plante, B. & Benzaazoua, M. (2019) Environmental impact of mine exploitation: An early predictive methodology based on ore mineralogy and contaminant speciation. Minerals, 9(7), 397. Available from: https://doi.org/10.3390/min9070397CrossrefCASWeb of Science®Google Scholar
- Cline, C., Hoksberg, A., Abry, R. & Janssen, A. (2003) Biological process for H2S removal from gas streams: The Shell-Paques/THIOPAQ™ gas desulfurization process. Proceedings of the Laurance Reid gas conditioning conference, 1– 18.Google Scholar
- Cordell, D., Drangert, J.O. & White, S. (2009) The story of phosphorus: Global food security and food for thought. Global Environmental Change, 19(2), 292– 305. Available from: https://doi.org/10.1016/j.gloenvcha.2008.10.009CrossrefWeb of Science®Google Scholar
- Cordell, D., Rosemarin, A., Schröder, J.J. & Smit, A.L. (2011) Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere, 84(6), 747– 758. Available from: https://doi.org/10.1016/j.chemosphere.2011.02.032CrossrefCASPubMedWeb of Science®Google Scholar
- De Crisci, A.G., Moniri, A. & Xu, Y. (2019) Hydrogen from hydrogen sulfide: Towards a more sustainable hydrogen economy. International Journal of Hydrogen Energy, 44(3), 1299– 1327.CrossrefCASWeb of Science®Google Scholar
- Dunn, J.B., Gaines, L., Kelly, J.C., James, C. & Gallagher, K.G. (2015) The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy and Environmental Science, 8(1), 158– 168. Available from: https://doi.org/10.1039/c4ee03029jCrossrefCASWeb of Science®Google Scholar
- Durmus, Y.E., Zhang, H., Baakes, F., Desmaizieres, G., Hayun, H., Yang, L. et al. (2020) Side by side battery technologies with lithium-ion based batteries. Advanced Energy Materials, 10(24), 1– 21. Available from: https://doi.org/10.1002/aenm.202000089Wiley Online LibraryWeb of Science®Google Scholar
- Essential Chemical Industry. (2016) Sulfuric Acid, essentialchemicalindustry.org. Available from: https://www.essentialchemicalindustry.org/chemicals/sulfuric-acid.html. Accessed August 2022.Google Scholar
- Grennfelt, P., Engleryd, A., Forsius, M., Hov, Ø., Rodhe, H. & Cowling, E. (2020) Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio, 49, 849– 864. Available from: https://doi.org/10.1007/s13280-019-01244-4CrossrefCASPubMedWeb of Science®Google Scholar
- Han, F., Fei, Y. & Cui, Z. (2016) Industrial metabolism of copper and sulfur in a copper-specific eco-industrial park in China. Journal of Cleaner Production, 133, 459– 466.CrossrefCASWeb of Science®Google Scholar
- Harper, G., Sommerville, R., Kendrick, E., Driscoll, L., Slater, P., Stolkin, R. et al. (2019) Recycling lithium-ion batteries from electric vehicles. Nature, 575(7781), 75– 86. Available from: https://doi.org/10.1038/s41586-019-1682-5CrossrefCASPubMedWeb of Science®Google Scholar
- Herrington, R. (2021) Mining our green future. Nature Reviews: Materials, 6, 456– 458. Available from: https://doi.org/10.1038/s41578-021-00325-9CrossrefWeb of Science®Google Scholar
- Hockenos, P. (2022) Will Russia’s war spur Europe to move on green energy? Available from: https://e360.yale.edu/features/will-russias-war-spur-europe-to-move-on-green-energy. Accessed August 2022.Google Scholar
- IEA. (2021a) The role of critical minerals in clean energy transitions. Paris, France: IEA. Available from: https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions. Accessed August 2022.Google Scholar
- IEA. (2021b) Net zero by 2050A roadmap for the global energy sector. Paris, France: IEA. Available from: https://www.iea.org/reports/net-zero-by-2050. Accessed August 2022.Google Scholar
- IPCC. (2018) 1.5°C global warming report. Available from: https://www.ipcc.ch/sr15/. Accessed August 2022.Google Scholar
- IPCC. (2022) Climate change 2022: Mitigation of climate change Available from: https://www.ipcc.ch/report/sixth-assessment-report-working-group-3/. Accessed August 2022.Google Scholar
- Kutney, G. (2013) Sulfur: History, technology, applications & industry. Toronto: ChemTec. Illustrated edition p250.Google Scholar
- Lewis, S.L. & Maslin, M.A. (2018) The human planet. London: Penguin, p. 465.Google Scholar
- Liu, J., Wang, J., Chen, Y., Shen, C.-C., Jiang, X., Xie, X. et al. (2016) Thallium dispersal and contamination in surface sediments from South China and its source identification. Environmental Pollution, 213, 878– 887.CrossrefCASPubMedWeb of Science®Google Scholar
- Ma, B., Yang, W., Yang, B., Wang, C., Chen, Y. & Zhang, Y. (2015) Pilot-scale plant study on the innovative nitric acid pressure leaching technology for laterite ores. Hydrometallurgy, 155, 88– 94.CrossrefCASWeb of Science®Google Scholar
- Martin, A. & Iles, A. (2020) The ethics of rare earth elements over time and space. HYLE-International Journal for Philosophy of Chemistry, 26(1), 5– 30.CASGoogle Scholar
- McGill, S.M. (2012) “Peak” phosphorus? The implications of phosphate scarcity for sustainable investors. Journal of Sustainable Finance and Investment, 2(3–4), 222– 239. Available from: https://doi.org/10.1080/20430795.2012.742635CrossrefGoogle Scholar
- McKinsey and Company. (2018) Litio y cobalto. London: McKinsey & Company Metals and Mining. Available from: https://www.mckinsey.com/~/media/mckinsey/industries/metalsandmining/our-insights/lithium-and-cobalt-a-tale-of-two-commodities/lithium-and-cobalt-a-tale-of-two-commodities.ashx. Accessed August 2022.Google Scholar
- Muyzer, G. & Stams, A.J. (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nature Reviews Microbiology, 6(6), 441– 454.CrossrefCASPubMedWeb of Science®Google Scholar
- Ober, J.A. (2002) Materials flow of sulfur. Reston, Va.: U.S. Dept. of the Interior, U.S. Geological Survey. Available from: https://pubs.usgs.gov/of/2002/of02-298/CrossrefGoogle Scholar
- Schnug, E. & Lottermoser, B.G. (2013) Fertilizer-derived uranium and its threat to human health. Environmental Science & Technology, 47, 2433– 2434.CrossrefCASPubMedWeb of Science®Google Scholar
- Shah, R. (2019) Global sulfuric acid market to surpass 324.1 million tons by 2027. New York, NY: Coherent Market Insights, Bloomberg. Available from: https://www.bloomberg.com/press-releases/2019-11-22/global-sulfuric-acid-market-to-surpass-324-1-million-tons-by-2027-coherent-market-insights. Accessed August 2022.Google Scholar
- Shiryaevskaya, A. & V. Dezem (2020) Decline in natural gas demand may take years to reset Available from: https://www.worldoil.com/news/2020/6/10/decline-in-natural-gas-demand-may-take-years-to-reset. Accessed August 2022.Google Scholar
- Speight, J. (2019) Chapter 8 – gas cleaning processes. In: Natural gas (second edition) a basic handbook, pp. 277– 324. Elsevier, Holland. Available from: https://doi.org/10.1016/B978-0-12-809570-6.00008-4Google Scholar
- Statista. (2020) Market size of sulfuric acid worldwide in 2015 and 2021. Statista.com. Available from: https://www.statista.com/statistics/961422/global-sulfuric-acid-market-volume/. Accessed August 2022.Google Scholar
- Tagliaferri, C., Evangelisti, S., Acconcia, F., Domenech, T., Ekins, P., Barletta, D. et al. (2016) Life cycle assessment of future electric and hybrid vehicles: A cradle-to-grave systems engineering approach. Chemical Engineering Research and Design, 112, 298– 309. Available from: https://doi.org/10.1016/j.cherd.2016.07.003CrossrefCASWeb of Science®Google Scholar
- Thomson, D.W. (1995) Prelude to the Sulphur War of 1840: The Neapolitan perspective. European History Quarterly, 25, 163– 180. Available from: https://doi.org/10.1177/026569149502500201CrossrefWeb of Science®Google Scholar
- U.S. Bureau of Mines. (1927–34) Mineral Resources of the United States, 1924–31 Available from: http://minerals.usgs.gov/minerals. Accessed August 2022.Google Scholar
- U.S. Bureau of Mines. (1933–96) Minerals Yearbook, 1932–94 Available from: http://minerals.usgs.gov/minerals. Accessed August 2022.Google Scholar
- U.S. Geological Survey. (1901–27) Mineral resources of the United States, 1900–23 Available from: http://minerals.usgs.gov/minerals. Accessed August 2022.Google Scholar
- U.S. Geological Survey. (1995–present) Minerals Yearbook, v. I. Available from: http://minerals.usgs.gov/minerals. Accessed August 2022.Google Scholar
- USGS. (2022) Mineral Commodities Summaries 2022. Available from: https://pubs.er.usgs.gov/publication/mcs2022. Accessed August 2022.Google Scholar
- Wagenfeld, J.-G., al-Ali, K., Almheiri, S., Slavens, A.F. & Calvet, N. (2019) Sustainable applications utilizing sulfur, a by-product from oil and gas industry: A state-of-the-art review. Waste Management, 95, 78– 89.CrossrefPubMedWeb of Science®Google Scholar
- Withers, P.J.A., Elser, J.J., Hilton, J., Ohtake, H., Schipper, W.J. & van Dijk, K.C. (2015) Greening the global phosphorus cycle: How green chemistry can help achieve planetary P sustainability. Green Chemistry, 17(4), 2087– 2099. Available from: https://doi.org/10.1039/c4gc02445aCrossrefCASWeb of Science®Google Scholar
- Yara. (2020) How we make our fertilizer, Yara.com. Available from: https://www.yara.com/crop-nutrition/why-fertilizer/production-of-fertillizer/. Accessed August 2022.Google Scholar
- Zhang, Y., Zheng, H., Yang, Z., Liu, G. & Meirong, S. (2015) Analysis of the industrial metabolic processes for sulfur in the Lubei (Shandong Province, China) eco-industrial park. Journal of Cleaner Production, 96, 126– 138.CrossrefCASWeb of Science®Google Scholar